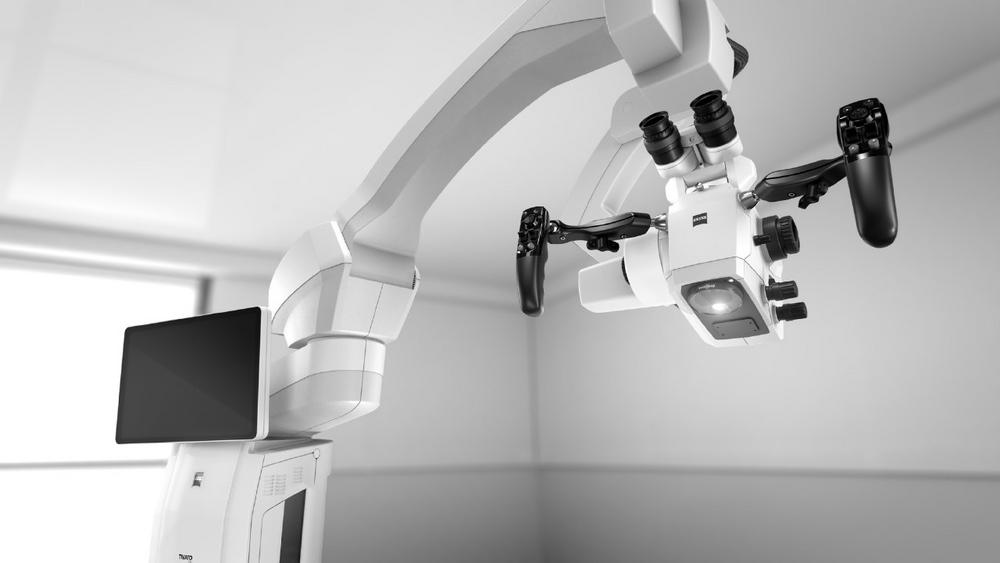
Workflow-Enhancing Visualization
TIVATO 700* expands the range of clinical applications for spine surgeons and gives the field of spine surgery access to cutting-edge technologies.
With Workflow-Enhancing Visualization, ZEISS TIVATO 700 makes fluorescence options for assessment of vessel patency and other applications available. Integrated 4K technology allows surgeons to visualize procedures in brilliant image quality. At the same time, assistants, OR staff and observers benefit from the outstanding resolution and image quality while following the surgical intervention – for an invaluable learning and training experience. “This system exceeded my expectations: much better view, more natural, better clarity, brighter. In addition, the working distance, especially, is much better,” Prof. Dr. Rene Schmidt, Klinik am Eichert, Göppingen, Germany.
Ultimate Reach & Flexibility
Different OR situations require medical devices to be highly adaptable. ZEISS TIVATO 700 meets these requirements with Ultimate Reach & Flexibility. An increased working distance offers spine surgeons and OR staff freedom of positioning including exoscopic and heads-up surgery, specifically when using long instruments. Excellent overhead clearance adds to this freedom allowing work underneath the system regardless of its placement.
All-Digital
Doctors and medical staff both benefit from the digital possibilities in ZEISS TIVATO 700, which offers a seamless entry into the world of fully connected visualization systems. ZEISS TIVATO 700 features an impressive, intuitive and easy-to-use graphical user interface for everyone in the operating room. The platform can be integrated into a hospital’s existing IT infrastructure supporting the digital workflow: image documentation, data management and data transfer can be easily managed along the surgical workflow using the ZEISS Connect App.
A connection to ZEISS Smart Services also enables improvements in service and maintenance. “Thanks to remote access, our service organization is able to identify and resolve issues quicker. Customers benefit from higher availability of their device,“ summarizes Dirk Brunner.
* Not available until conformity assessment according to MDD 93/42 is finished. Only for sale in selected countries. The shown contents may differ from the current status of approval of the product or service offering in your country.
Carl Zeiss Meditec AG (ISIN: DE 0005313704), the Medical Technology Business Group of ZEISS, is listed on SDAX and TecDAX of the German stock exchange and one of the world’s leading medical technology companies. The Company supplies innovative technologies and application- oriented solutions designed to help doctors improve the quality of life of their patients. The Company offers complete solutions, including implants and consumables, to diagnose and treat eye diseases. The Company creates innovative visualization solutions in the field of microsurgery. With approximately 3,000 employees worldwide, the Group generated revenue of € 1,189.9 million in fiscal year 2016/17 (to 30 September).
The Group’s head office is located in Jena, Germany, and it has subsidiaries in Germany and abroad; more than 50 percent of its employees are based in the USA, Japan, Spain and France. The Center for Application and Research (CARIn) in Bangalore, India and the Carl Zeiss Innovations Center for Research and Development in Shanghai, China, strengthen the Company’s presence in these rapidly developing economies. Around 41 percent of Carl Zeiss Meditec AG’s shares are in free float. The remaining approx. 59 percent are held by Carl Zeiss AG, one of the world’s leading groups in the optical and optoelectronic industries.
For more information visit our website at: www.zeiss.com/med
Carl Zeiss Meditec AG
Göschwitzer Strasse 51-52
07745 Jena
Telefon: +49 (3641) 220-0
http://www.meditec.zeiss.de
Director Investor Relations
Telefon: +49 (3641) 220-116
E-Mail: investors.meditec@zeiss.com
![]()