CV Magazine’s annual Corporate Excellence Awards are handpicked by their dedicated research team based purely on the comprehensive analysis of both qualitative and quantitative research during the past 12 months. The awards are designed to celebrate excellence in a number of core industries across the business landscape, highlighting those who have truly gone above and beyond to succeed in their endeavors, continually innovating, growing and improving.
Corporate Vision is created by a highly experienced and passionate team of business experts, advisors and insiders dedicated to shine a spot on the brightest, best performing and most deserving companies around the world by carefully analyzing what each industry has to offer. Their business experts recognize the very best and promote the success of companies committed to innovation that had the best performance over the past year.
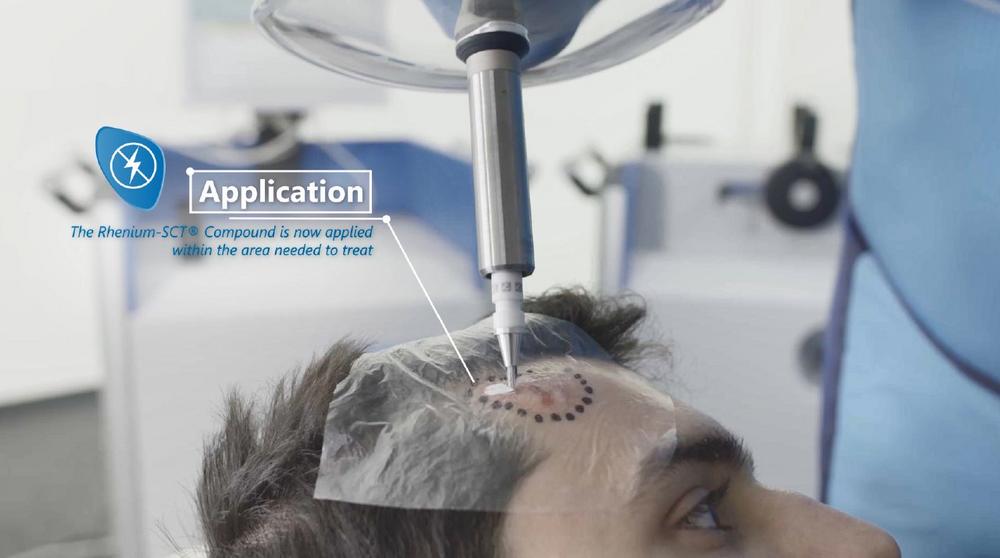
OncoBeta GmbH has been a gamechanger in the way non-melanoma skin cancer (NMSC) is treated. By always having the patient´s perspective in focus, OncoBeta is today helping redefine patient experience. “With our therapeutic for NMSC- the Rhenium-SCT® – OncoBeta offers a personalized, non-invasive and painless alternative for patients suffering from NMSC” -states Shannon D. Brown III, CEO and Managing Director of OncoBeta GmbH – The global incidence of NMSC has been drastically increasing over the past few decades. The standard care options for NMSC are either painful, non-aesthetically desired or invasive. “OncoBeta is part of the story trying to rectify this” -continues Shannon. With the Rhenium-SCT, OncoBeta is not only improving patient outcomes but is also ensuring scalability and patients access to this therapeutic solution.
For more information about the CV Magazine Awards:
https://www.cv-magazine.com/2019-aiming-to-improve-overall-patient-outcomes-and-experiences
About the Rhenium-SCT® (Skin Cancer Therapy)
The Rhenium-SCT® is a non-invasive, painless therapy generally providing for unparalleled aesthetic results, even in cases otherwise considered difficult to treat. The Rhenium-SCT® utilizes the radioisotope Rhenium-188 in an epidermal application with optimal properties for the treatment of NMSCs (non-melanoma skin cancers). Due to the specially designed devices and accessories the Rhenium-SCT® compound never comes in direct contact with the patients’ skin and the application is safe and simple for the applying physician. Most cases of non-melanoma skin cancers (Basal Cell Carcinomas and Squamous Cell Carcinomas) can be treated using the Rhenium-SCT® with a single application, applied in one single session. Scar-free healing of the treated lesion area and the regeneration of healthy tissue occurs usually within a few weeks after treatment.
About OncoBeta® GmbH
OncoBeta® GmbH with its headquarters located at the gate – Garching Technology and Founders Center near Munich, is a privately held medical device company, specializing in the development and commercialization of state-of-the-art, innovative therapies utilizing epidermal radioisotope applications. Since its foundation, OncoBeta® GmbH has concentrated its efforts on the development, regulatory approval(s) and commercialization of the epidermal radioisotope therapy Rhenium-SCT® (Skin Cancer Therapy), targeting non-melanoma skin cancers. Since then, OncoBeta® has successfully perfected the customized application and device management system in conformity with all health, safety, and environmental protection regulatory standards. OncoBeta`s outstanding work has already been rewarded with several international recognitions such as “Top 20 MedTech Outlook Solution Provider in Europe”, “Top 30 Ethical Companies of the Year 2019” by The Silicon Review Magazine and the “Top 100 Red Herring Europe Award”. The Rhenium-SCT® is European CE certified and is filed for regulatory approval in several other countries outside the European Union.
Find out more about the Rhenium-SCT® at https://www.oncobeta.com
Forward-looking statements
This announcement includes forward-looking statements that involve risks, uncertainties and other factors, many of which are outside of OncoBeta’s control and which could cause actual results to differ materially from the results discussed in the forward-looking statements. Forward-looking statements include statements concerning OncoBeta’s plans, objectives, goals, future events, performance and/or other information that is not historical information. All such forward-looking statements are expressly qualified by these cautionary statements and any other cautionary statements which may accompany the forward-looking statements. OncoBeta® undertakes no obligation to publicly update or revise forward-looking statements to reflect subsequent events or circumstances after the date made, except as required by law.
gate Garchinger Technologie- und Gründerzentrum GmbH
Lichtenbergstraße 8
85748 Garching bei München
Telefon: +49 (89) 5484-0
Telefax: +49 (89) 5484-1010
http://www.gategarching.com
Marketing & Öffentlichkeitsarbeit
Telefon: +49 (89) 5484-1110
Fax: +49 (89) 5484-1010
E-Mail: lisa.hyna@gategarching.com
![]()