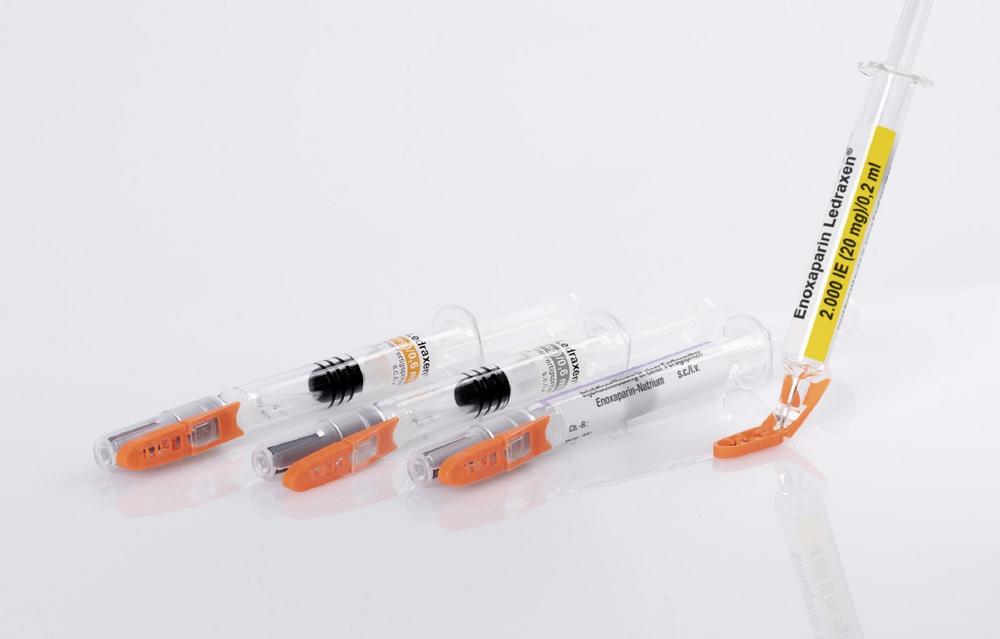
Kingfriend offers its anticoagulant medication for the prevention of thrombosis as an enoxaparin-sodium injection solution in prefilled syringes. For the European market launch, the company was looking for a cost-efficient needle protection solution that meets all legal requirements.
The pharmaceutical company based in Nanking found the suitable solution in Schreiner MediPharm’s Needle-Trap. The needle protection label with an integrated trap marks the syringe and helps to protect healthcare staff against needlestick injuries. It meets the requirements of EU Directive 2010/32/EU for protection against injuries and DIN EN ISO 23908 for protection against sharps.
Kingfriend was especially convinced of the Needle-Trap design. The needle trap is directly connected to the label and features a particularly compact construction. Consequently, the secondary packaging for the heparin syringes does not have to be modified and saves warehousing and logistics costs. The production process requires only minimal adaptations. This is innovative and extremely economical.
The prefilled enoxaparin-sodium syringe with Needle-Trap was launched in Germany in the fall of 2020; the product is marketed via the partner company Venipharm.
About Kingfriend
Nanjing Kingfriend Biochemical Pharmaceutical Co., Ltd. is a Chinese public company based in Nanjing that is primarily engaged in research and development, production and sale of injectable products. Kingfriend is the global leading company in heparin and low molecular weight heparin (enoxaparin sodium) products. The company with almost 1,000 employees primarily exports its products to Europe and the United States. Its customer base includes the top 500 pharmaceutical companies.
Schreiner MediPharm, a business unit of Schreiner Group GmbH & Co. KG based in Oberschleissheim near Munich, is a leading developer and manufacturer of innovative, multifunctional specialty labels and marking solutions with value-added benefits for the healthcare industry. Thanks to its strong solutions expertise and specialized know-how Schreiner MediPharm is a highly capable development partner and reliable quality supplier to leading pharmaceutical and medical device technology companies worldwide.
Schreiner Group GmbH & Co. KG
Bruckmannring 22
85764 Oberschleißheim
Telefon: +49 (89) 31584-0
Telefax: +49 (89) 31584-5166
http://www.schreiner-group.com
Produktkommunikation
Telefon: +49 (89) 31584-5674
E-Mail: andrea.richter@schreiner-group.com
![]()